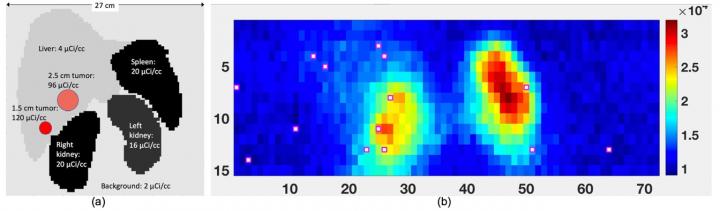
IMAGE: a) Maximum intensity projection showing relative positions of organs and tumors in the simulations with sample activity levels. b) Plot of counts for 2D detector array. Red boxes represent detector… view more
Credit: R. Miyaoka et al., University of Washington, Seattle, WA
Researchers at the University of Washington in Seattle, Washington, are developing a user-friendly (worn at home) vest with technology that collects data to tailor personalized therapy for patients with metastatic, somatostatin-receptor-2 positive neuroendocrine tumors (NETs). The study was presented at the 2019 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI).
Targeted therapy using lutetium-177 (177Lu)-DOTATATE greatly increases progression-free survival for NETs patients. While approved by the United States Food and Drug Administration (FDA), the FDA package instructions call for patients to receive a standardized protocol, regardless of size or weight. Traditionally, targeted radionuclide therapies are personalized based on dose to the main organs at risk (OAR, e.g., kidneys, liver, spleen).
“Organ specific dosimetry for the 177Lu-DOTATATE (Lutathera) is the norm at many medical centers outside of the United States,” explains Robert Miyaoka at the University of Washington. “Longitudinal imaging studies are conducted after each therapy treatment to determine the cumulative dose to the OAR for each patient. The standardized 177Lu-DOTATATE treatment protocol in the United States consists of four 200 mCi doses spaced two months apart. Although this is safe for a vast majority of patients, it is less than optimal for most. Studies out of Europe are revealing that tailoring the number of treatment doses based upon the dose-limiting toxicity to the patient’s OAR can more than double the progression-free and overall survival for NET patients undergoing 177Lu-DOTATATE therapy.”
Another factor the researchers sought to address is the fact that traditional imaging-based methods for organ dosimetry estimation for 177Lu require three-to-four longitudinal imaging sessions spread over seven days. This is expensive, uses a lot of clinic resources and is burdensome to the patient.
“We propose to create a lightweight, low-cost, wearable, patient-specific technology that will allow organ-specific measurement recordings to be made within the comfort of the patient’s home,” Miyaoka says. “The garment [called a multi-detector personalized home dosimetry (MD PHD) vest] will house 15-20 small radiation detectors, strategically placed within the vest based upon the patient’s own anatomy. In addition to the radiation detectors the vest will be coupled to a compact electronics pack that will acquire the data and send it via WiFi or cellular services to a secure website where medical personnel/software can check the data for quality control in near real-time.”
He further explains, “The patient will be asked to wear the vest for a two-minute data acquisition once a day for seven (and up to 21) days. Based upon these at-home measurements and a single SPECT/CT image taken 24 hours after the therapy administration, organ specific dosimetry will be determined for all of the patient’s OAR.” With the information collected via the vest, physicians would be able to tailor the number of treatments based upon personalized organ dosimetry information.
Miyaoka reports, “Preliminary vest results from simulations are showing that at-home vest measurements made over 7-21 days can provide organ-specific washout rates with precision as good or better than the current accepted gold standard of three-four quantitative SPECT/CT images acquired over seven days. The initial goal of this technology is to enable personalized 177Lu-DOTATATE therapies in the United States and to lower the cost for treatment personalization throughout the world.”
###
Abstract 313: “Wearable Technology to Enable Personalization of Lu177-DOTATATE Therapy for Neuroendocrine Tumor Patients,” Robert Miyaoka, Larry Pierce, Robert Harrison and Hubert Vesselle, University of Washington, Seattle, WA. SNMMI’s 66TH Annual Meeting, June 22-25, 2019, Anaheim, CA.
To schedule an interview with the researchers, please contact David Harrison at (410) 804-1728 or david@harrisoncommunications.net. All 2019 SNMMI Annual Meeting abstracts can be found online at //jnm.
About the Society of Nuclear Medicine and Molecular Imaging
The Society of Nuclear Medicine and Molecular Imaging (SNMMI) is an international scientific and medical organization dedicated to advancing nuclear medicine and molecular imaging, vital elements of precision medicine that allow diagnosis and treatment to be tailored to individual patients in order to achieve the best possible outcomes.
SNMMI’s more than 16,000 members set the standard for molecular imaging and nuclear medicine practice by creating guidelines, sharing information through journals and meetings and leading advocacy on key issues that affect molecular imaging and therapy research and practice. For more information, visit //www.
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.

