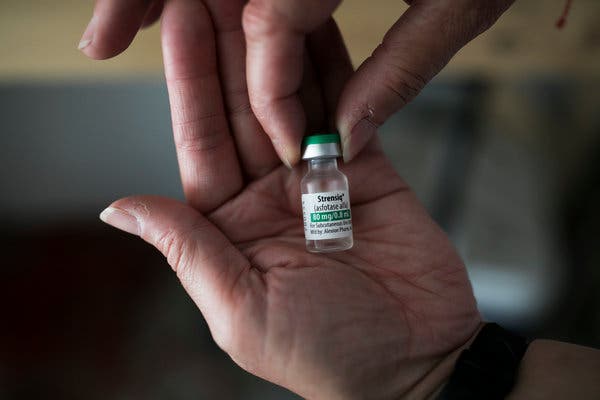
PERRYSBURG, Ohio — Dawn Patterson keeps a multimillion-dollar drug in the fridge, next to a bottle of root beer and a jar of salsa.
The drug, Strensiq, treats a rare bone disease that afflicted her with excruciating pain and left her struggling to work or care for her family.
A year after she began taking the drug, Ms. Patterson, 49, credits it with nearly vanquishing her pain, enabling her to return to work part time for a hospital.
But her family and her husband’s union, which covers the drug’s cost, have been shocked by the mounting bills for the treatments for her and two of her children, who have the same genetic disease. In 2018, the union faced a potential $6 million annual bill for the Patterson household, casting doubt over the future of the labor group’s generous drug coverage and the family’s health.
“My jaw really did drop,” Ms. Patterson said. “I was like, what? This is crazy.”
While it’s hardly a household name, Strensiq is one of the costliest drugs in the world. It is part of an unsettling trend in which ultraexpensive drugs are becoming more common, spurring a national debate over whether any drug should cost millions of dollars, and whether Americans will be priced out of lifesaving treatments as drug companies maximize their profits.
Extraordinary scientific advances — as well as tax breaks and other government incentives for developing drugs for rare diseases — have spawned treatments, and even cures, for illnesses that were once a death sentence. And now that cheaper generic drugs account for about 90 percent of all prescriptions filled in the United States, pharmaceutical companies are turning to rare-disease treatments and gene therapies as their next profit engine, with major companies like Pfizer and Novartis investing in drugs for tiny pools of patients.
These drugs face no real competition, including from generic companies, leaving the door wide open for manufacturers to set nearly any price they want. How high is too high a cost, some argue, if a drug can save 200 or 300 babies a year from a debilitating, degenerative illness or death?
Rare diseases, however, aren’t all that rare. There are an estimated 7,000 of them, and about 30 million Americans have one — roughly the same number of people in the United States with diabetes. And although there are no treatments for most rare diseases, new therapies are coming on the market nearly every month, with some reaching beyond $2 million a year for a single treatment. Of 59 new drugs approved in 2018, more than half, or 34, were for rare diseases. Those treatments are typically the most expensive, helping to drive an increase in overall spending on prescriptions nationwide.
As more and more families are beseeching drug companies and insurers to pay for this novel class of treatments, both big and small employers are getting hit with higher drug bills. It may be for a worker’s child with hemophilia whose treatments can cost over $1 million or for an employee receiving immunotherapy for lung cancer. But not every union or corporate employer has an adequate cushion to absorb these prescription bills.
“In the past, we’d think of rare and orphan diseases and an employer would say, oh, I just had bad luck — I had one of these patients,” said Dr. Steve Miller, the chief clinical officer of Cigna, which owns Express Scripts, a pharmacy benefit manager that negotiates with drug companies on behalf of employers to cover prescriptions for 75 million Americans. “Going forward, if we’re successful and have therapies for these things, everyone’s going to have them.”
The Patterson family’s experience also exposes a stunning lack of transparency in drug pricing; many rare-disease drugs are priced based on a patient’s weight, meaning a prescription for an adult costs many times more than one for a child or infant. Pharmaceutical companies often obscure the real cost with initial estimates — provided to the media or Wall Street analysts — that are far less than the eventual real-world cost of the medicines.
In the Pattersons’ case, Strensiq was expected to cost about $285,000 in 2015 according to its manufacturer, Alexion Pharmaceuticals. But here’s the catch: That price was based on the assumption that most patients would be children or infants and would weigh an average of about 50 pounds. In addition, Alexion would not discuss how much it would cost adults. In 2018, Ms. Patterson’s drug bill approached $2 million.
The breathtaking price threw the labor union — the International Brotherhood of Boilermakers, which covers her health care through her husband, Bill — into a crisis.
Four months after she began taking Strensiq, the union’s health plan put payment for the drug on hold to evaluate whether she really needed it.
“This is the only choice of medication that we have,” Ms. Patterson recalled telling the union. “There are no other options here, and we need this medicine.”
Still, the toll on the union’s health plan is astonishing: At one point in 2018, for every hour that one of the union’s 16,000 members worked, 35 cents of his or her pay went to Alexion to cover the Pattersons’ prescriptions.
“It’s an alarm that goes off when you see this,” said Lori Jasperson, who oversees the health care plan for the union’s workers and their families. Boilermakers keep the country’s industrial plants running, maintaining and building equipment in everything from oil refineries to shipbuilding factories.
Because Strensiq needs to be taken indefinitely by patients who need it, Ms. Jasperson estimated that the Pattersons’ total drug coverage could cost the union $60 million.
Alexion, which focuses on treatments for rare diseases and is based in Boston, declined several requests for an interview. Ultimately, under pressure from the boilermakers’ union and Express Scripts and questioned by The New York Times, the company told Express Scripts that it would cap the annual cost at $1.5 million for each adult in commercial plans covered by the pharmacy benefits manager, including the union’s. Alexion said that it is negotiating similar price caps with others, but that those talks were confidential.
In a statement, Alexion defended its prices, saying that developing medicines for rare diseases is complex, costly and carries a significant risk of failure. “Society can’t lose focus on very small populations of several hundred people,” it said.
Presidential candidates and members of Congress have railed against drug companies in recent years, but have largely overlooked the spate of million-dollar treatments for uncommon illnesses or cancers.
Employers and unions say the impact is already being felt. Some small businesses, hit with just a single claim for a family like the Pattersons, have considered ending their employee health coverage. Others have drastically cut back coverage for drugs, and some employers are considering excluding coverage for expensive and novel treatments like gene therapy.
The number of these claims is climbing. Sun Life, which provides specialized insurance to employers to cover their most expensive medical bills, saw claims over $1 million increase by a third over three years, reaching 203 claims in 2018.
Companies cannot predict these costs. “You are one hire, one diagnosis away from this happening to you,” said Rich Fuerstenberg who advises employers for the consulting firm Mercer. “It’s literally like being struck by lightning.”
‘A Slow-Moving Train Collision’
Drug companies once shunned investment in rare diseases because, with such a small market, there was little incentive. But that began to change after the passage of the Orphan Drug Act, a 1983 law that provides government subsidies for clinical trials as well as tax incentives and additional monopoly protection to companies that develop products for rare diseases.
Scientific advancements have further propelled the field as researchers have begun to crack the code on many deadly genetic diseases. It’s a heady time for people with serious diseases, but one that also breeds anxiety as they worry about paying.
Maria Kefalas, a patient advocate whose daughter, Calliope, has a rare form of leukodystrophy, described the situation as a “slow-moving train collision.”
At least two companies are developing gene therapies for leukodystrophy, a deadly group of genetic disorders that cause damage to the brain and central nervous system, robbing children of the ability to crawl, walk, swallow and speak. Treatments, Ms. Kefalas worries, will cost millions of dollars. “And I’m sitting here, as a mom, looking at working with families and saying, ‘I don’t know who is going to pay for this.’”
Some attribute the trend of drug companies charging very high prices to the Affordable Care Act, the federal law that banned lifetime caps and annual limits on patients’ coverage. It includes a mandate that employers keep paying for drugs, no matter the cost.
“There was no market for this,” Mr. Fuerstenberg said. “Now there is.”
Because these drugs are one of a kind, companies like Alexion hold all the leverage. They offer minimal discounts and control the price.
Dr. Jonathan Gavras, the chief medical officer of the pharmacy benefit manager Prime Therapeutics, recalled a small, self-insured employer that was forced to cover a worker who needed Strensiq a few years ago. Paying for the employee’s drug, he said, “essentially almost put the group under.”
The Pattersons’ bills forced the boilermaker union’s leaders to consider raising its workers’ premium contributions for the first time in eight years.
Ms. Jasperson sought help from officials — reaching out to her congressman, the Labor Department and even President Trump, who has made consumer drug prices a major talking point.
“The consequence of Alexion charging this rapacious amount for our boilermaker families to have access to this medication forces our plan to either not cover this medication at all, or consider putting limits on all specialty medications going forward,” Ms. Jasperson wrote in a letter to Mr. Trump.
Hidden Costs, Unexpected Increases
Dosages based on weight are not unique to Alexion. For example, Exondys 51, a treatment for a rare form of muscular dystrophy sold by Sarepta Therapeutics, was supposed to run about $300,000 a year when it was approved in 2016, according to the company’s statements to analysts at the time. But the annual cost is closer to $1 million, according to an analysis by Express Scripts.
Lumizyme, for people with Pompe disease, an inherited enzyme disorder that can prove fatal in infants, costs nearly double the $300,000 its manufacturer, Genzyme — later acquired by Sanofi — said it would likely cost when it was approved in 2010. Drugs for hemophilia and other rare diseases are similarly pricey.
Sarepta, Sanofi and other manufacturers of rare-disease drugs say they provide assistance to patients who are uninsured or can’t afford the out-of-pocket costs of their products. The companies say they have to recoup their investment in drugs that treat a small pool of patients — rare diseases are defined as affecting fewer than 200,000 people nationwide. The Pattersons’ disease, hypophosphatasia, is believed to affect just 1,300 people in the United States.
But even a treatment for hundreds of people can become a billion-dollar product. Alexion acquired Strensiq in 2012, when it bought Enobia Pharma for about $1.1 billion. Strensiq has since generated about $1.3 billion in revenue for Alexion — including $271 million in the first half of 2019 alone.
Strensiq is not even Alexion’s top-selling drug. Another drug, Soliris, for rare immune and blood disorders, brought in more than $3.5 billion last year and regularly ranks among the world’s most expensive drugs.
Alexion said it invested in Strensiq by completing clinical trials and ensuring an adequate supply. “There continues to be significant ongoing investment required to bring Strensiq to patients, including in diagnostic and educational efforts and manufacturing,” the company said in its statement.
The value of Strensiq to patients can vary. It can seem like a miracle for babies with severe forms of hypophosphatasia, whose bones are so soft that sometimes they are barely visible on X-rays. Strensiq replaces a deficient enzyme, alkaline phosphatase, and allows the bones to mineralize. Before Strensiq, many infants born with the disease died within months. Now, they can live near-normal lives.
In adults like Ms. Patterson, who have less severe forms of the disease and whose costs are highest, Strensiq can significantly improve their quality of life.
Struggling to Find Payment Options
Many of the Congressional proposals aimed at lowering drug prices would do nothing for ultraexpensive drugs like Strensiq. In an effort to soften public outrage over high prices, some drug makers have started to offer other options.
When Novartis announced that Zolgensma, its gene therapy treatment that can halt the progression of spinal muscular atrophy in babies, would cost $2.1 million, the company offered a pay-over-time plan. It said insurers and employers would be permitted to pay in installments over three to five years, and that some of the money could be refunded if the treatment failed to work.
But some note that the drug makers retain autonomy over the price. “A lot of these products haven’t hit the market yet, and there’s still a hope we can do something about the price rather than try to figure out a 20-year mortgage payment for them,” said Matt Salo, executive director of the National Association of Medicaid Directors.
The new payment options also don’t address drugs like Strensiq, which patients will need for the rest of their lives.
Ms. Patterson learned that she had the condition when she was undergoing unrelated tests a few years ago, and then her children were tested. She doesn’t know if other relatives have the disease, but recalled that one of her great-grandmothers was bedridden through much of her childhood.
She worries about the impact of her family’s medical situation on the boilermakers’ union. Bill Patterson, 47, has been a member for more than 20 years.
He “would gladly pay for any other person’s family, their wife, their children, because that’s what boilermakers do,” she said. But “that’s a lot of money.”
“And why?” she said. “Why does it have to be that much? I don’t understand that.”
One Sunday, Ms. Patterson puttered around her kitchen, stirring ground beef for tacos and checking on a queso dip in the slow cooker. Before she began taking Strensiq, Ms. Patterson had all but stopped cooking for her family.
Her daughter, Melissa Mason, said she began experiencing pain and fatigue about three years ago, when she turned 20. “I was helping my grandmother with some yard work for a few days and I could barely help her,” she said. “It seemed like she could do more than I could.”
Ms. Patterson’s son, Will, is 18 and has virtually no symptoms. He played first base for his high school baseball team and plans to play baseball at Adrian College in Michigan next year.
At the end of last year, he decided to stop taking Strensiq, reducing the cost of the family’s treatments for each boilermaker to 26 cents an hour.
Ms. Mason, who is working part time as a restaurant dishwasher, wonders how she will stay on the medicine three years from now when she turns 26 and will no longer be covered under her parents’ insurance.
“When it hits that point, I don’t know if I’ll be able to take it anymore,” she said. “I don’t know if that will be possible.”
[Like the Science Times page on Facebook. | Sign up for the Science Times newsletter.]

