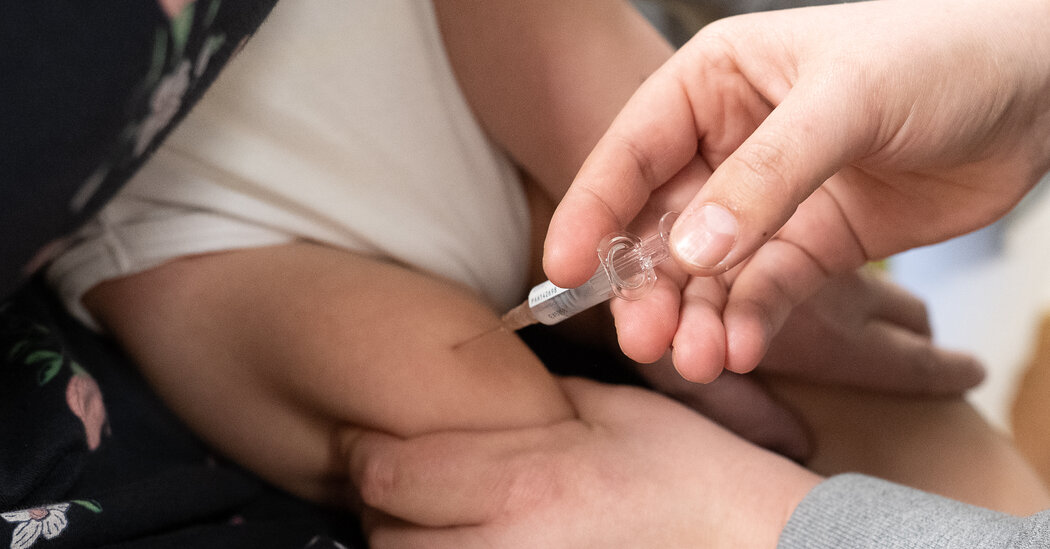
This winter, for the first time ever, there were two vaccines available to ward off respiratory syncytial virus, which is particularly dangerous to older adults and infants. Only one of them — Abrysvo, made by Pfizer — was approved for pregnant women, and neither was for young children.
The distinction apparently slipped by some clinicians and pharmacists.
At least 128 pregnant women were mistakenly given the alternative vaccine — Arexvy, by GSK — and at least 25 children under age 2 received a vaccination, the Centers for Disease Control and Prevention has warned.
Dr. Sarah Long, a pediatric infectious disease physician and an adviser to the agency, said she was “blindsided” by the reports. “It is very upsetting that this should happen,” she said.
Arexvy has not been tested in pregnant women or children, so information about its effects in those groups is limited. No serious harms from the errors have yet been confirmed, but the outcome was unknown in a majority of reported cases.
Based on available data, Dr. Long said she was more concerned about the young children who received an R.S.V. vaccine than the pregnant women who received Arexvy or their babies. Evidence from animal testing “strongly suggests” that Arexvy might exacerbate R.S.V. infection in children younger than 2, rather than mitigate it, according to the Food and Drug Administration.
To prevent that, the C.D.C. has recommended that the children who mistakenly got either vaccine also be given nirsevimab (sold as Beyfortus), a monoclonal antibody that provides strong immune protection, while the R.S.V. season lasts.
