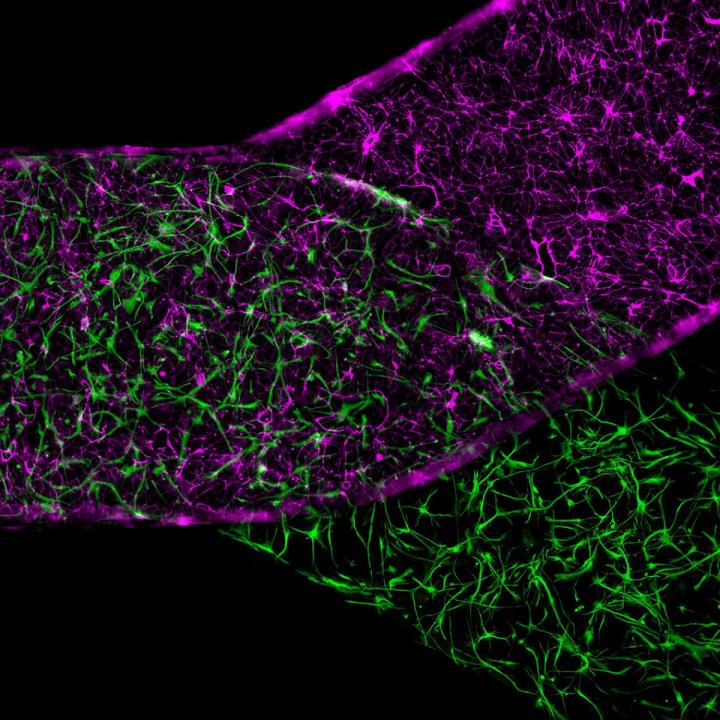
IMAGE: Organ-Chip recreates the microenvironment that cells require to exhibit an unprecedented level of biological function and to behave like they do in the human body. view more
Credit: Emulate, Inc.
LOS ANGELES (June 6, 2019) — Scientists can’t make a living copy of your brain outside your body. That’s the stuff of science fiction. But in a new study, they recreated a critical brain component, the blood-brain barrier, that functioned as it would in the individual who provided the cells to make it. Their achievement – detailed in a study published today in the peer-reviewed journal Cell Stem Cell – provides a new way to make discoveries about brain disorders and, potentially, predict which drugs will work best for an individual patient.
The blood-brain barrier acts as a gatekeeper by blocking toxins and other foreign substances in the bloodstream from entering brain tissue and damaging it. It also can prevent potential therapeutic drugs from reaching the brain. Neurological disorders such as amyotrophic lateral sclerosis (Lou Gehrig’s disease), Parkinson’s disease and Huntington’s disease, which collectively affect millions of people, have been linked to defective blood-brain barriers that keep out biomolecules needed for healthy brain activity.
For their study, a team led by Cedars-Sinai investigators generated stem cells known as induced pluripotent stem cells, which can produce any type of cell, using an individual adult’s blood samples. They used these special cells to make neurons, blood-vessel linings and support cells that together make up the blood-brain barrier. The team then placed the various types of cells inside Organ-Chips, which recreated the body’s microenvironment with the natural physiology and mechanical forces that cells experience within the human body.
The living cells soon formed a functioning unit of a blood-brain barrier that functions as it does in the body, including blocking entry of certain drugs. Significantly, when this blood-brain barrier was derived from cells of patients with Huntington’s disease or Allan-Herndon-Dudley syndrome, a rare congenital neurological disorder, the barrier malfunctioned in the same way that it does in patients with these diseases.
While scientists have created blood-brain barriers outside the body before, this study further advanced the science by using induced pluripotent stem cells to generate a functioning blood-brain barrier, inside an Organ-Chip, that displayed a characteristic defect of the individual patient’s disease.
The study’s findings open a promising pathway for precision medicine, said Clive Svendsen, PhD, director of the Cedars-Sinai Board of Governors Regenerative Medicine Institute. “The possibility of using a patient-specific, multicellular model of a blood-brain barrier on a chip represents a new standard for developing predictive, personalized medicine,” he said. Svendsen, professor of Medicine and Biomedical Sciences, was the senior author of the study.
The research combined the innovative stem cell science from investigators at Cedars-Sinai in Los Angeles with the advanced Organs-on-Chips technology of Emulate, Inc. in Boston. Emulate’s Human Emulation System recreates the microenvironment that cells require to exhibit an unprecedented level of biological function and to behave like they do in the human body. The system consists of instrumentation, software apps, and Organ-Chips, about the size of AA batteries, with tiny fluidic channels lined with tens of thousands of living human cells.
The co-first authors of the study are Gad Vatine, PhD, from Ben-Gurion University of the Negev in Beer Sheva, Israel, a former postdoctoral scientist at Cedars-Sinai; Riccardo Barrile, PhD, of Emulate, a former postdoctoral fellow at Cedars-Sinai; and Michael Workman, a PhD student in the Cedars-Sinai Graduate School of Biomedical Sciences.
The research is one of several collaborative projects involving Cedars-Sinai and Emulate, Inc., which In February 2018 announced a joint Patient-on-a-Chip program to help predict which disease treatments would be most effective based on a patient’s genetic makeup and disease variant. The program is an initiative of Cedars-Sinai Precision Health, whose goal is to drive the development of the newest technology and best research, coupled with the finest clinical practice, to rapidly enable a new era of personalized health.
###
Disclosure: Cedars-Sinai owns a minority stock interest in Emulate, Inc. An officer of Cedars-Sinai serves on Emulate’s board of directors. Emulate provided no financial support for this research. Six of the study’s authors are employees and shareholders of Emulate.
Funding: Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke and the National Center for Advancing Translational Sciences of the National Institutes of Health under award number 1UG3NS105703, the California Institute for Regenerative Medicine, The ALS Association, the Sherman Family Foundation and the Israel Science Foundation.
DOI: 10.1016/j.stem.2019.05.011
Read more on the Cedars-Sinai Blog: What Are Induced Pluripotent Stem Cells?
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.

