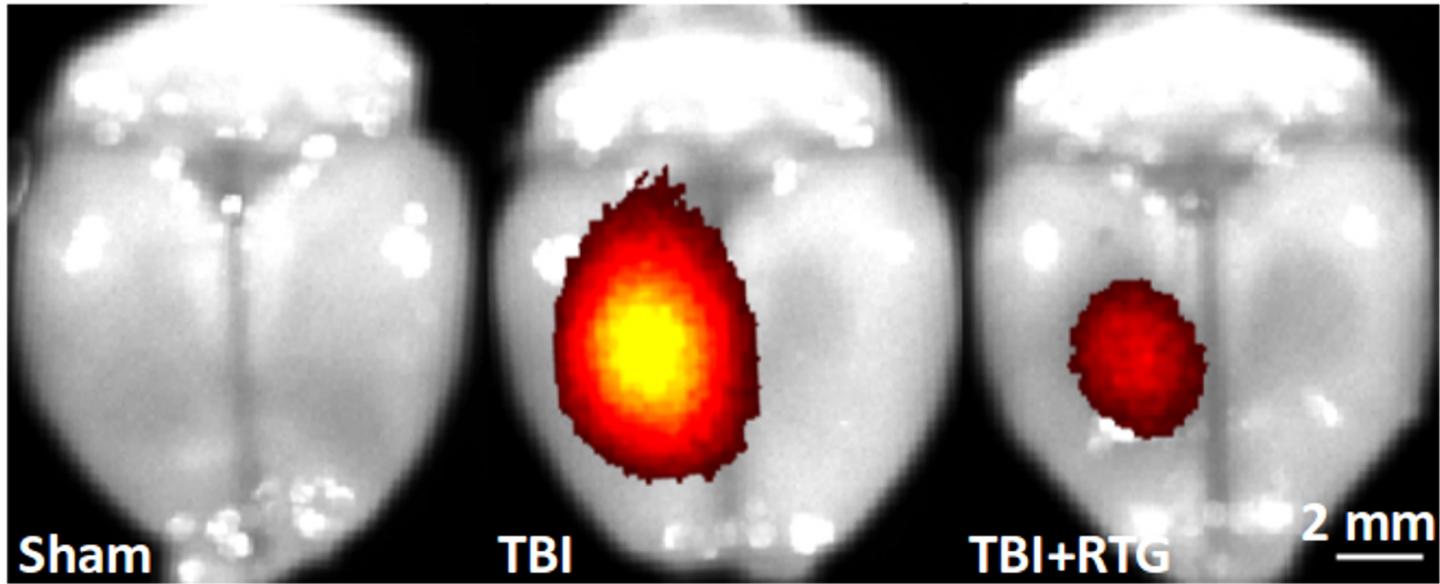
IMAGE: Red and yellow areas show sites of cell death in the mouse brain. The brain at left has no traumatic brain injury (TBI). The brain at center is post-TBI. The… view more
Credit: Mark S. Shapiro, Ph.D., UT Health San Antonio
An experimental treatment given to mice after a traumatic brain injury (TBI) reduced damage almost to the levels of mice that never had a TBI, researchers at UT Health San Antonio reported. The study was published July 4 in the Journal of Cerebral Blood Flow and Metabolism.
The scientists hope to convert the discovery into a simple and effective treatment for use in emergency rooms or by first responders shortly after a TBI has occurred in military and civilian settings. Currently, no treatment options exist for TBI patients.
“After a traumatic brain injury, about 40% of mice experience a seizure within one week, and many continue to experience seizures for years, leading to epilepsy disease,” said study senior author Mark S. Shapiro, Ph.D., professor of cellular and integrative physiology at UT Health San Antonio. “This closely parallels what happens in human patients, followed by cognitive dysfunction and changes in emotional state.”
Damaging effects
After a TBI, dangerous inflammation occurs throughout the brain, causing nerve cells to die and the blood-brain barrier, which is critical to maintaining normal brain function, to break down, said lead author Fabio A. Vigil, Ph.D., postdoctoral fellow in Dr. Shapiro’s lab.
Preventing abnormal electrical activity
The novel therapy increases the activity of “M-type” KCNQ potassium ion channels, which are proteins that can halt uncontrolled electrical currents in nerve cells. Abnormal currents begin immediately after a TBI, even before a seizure has a chance to occur, and the therapy aims to counteract this, thus nipping in the bud this destructive chain of events.
“No seizures were observed in the treated mice whatsoever,” Dr. Vigil said.
Neurologist’s perspective
“We need treatments that alter some of the disabling consequences of TBI,” said study co-author Jose E. Cavazos, M.D., Ph.D., a neurologist and epilepsy specialist at UT Health San Antonio. “Current antiseizure medications don’t prevent the development of post-traumatic epilepsy. Our study examined this critically important therapeutic gap, and proposes a novel pharmacological intervention shortly after TBI that might prevent post-traumatic epilepsy.”
If such a therapy can be developed, it would be a game-changer for patients, Dr. Cavazos said. Approximately 6% of all epilepsy cases are caused by head trauma.
“Think about the possibility of taking a medication shortly after the injury and preventing disabling epileptic seizures months to years later,” Dr. Cavazos said.
Post-trauma impact
Study co-author Robert Brenner, Ph.D., of UT Health San Antonio, provided expertise in seizures and seizure monitoring. He said the study’s most important finding is that reducing excess electrical activity in the central nervous system via a therapy such as this has beneficial post-trauma effects that extend well beyond action as an anticonvulsant. These effects include reducing dangerous inflammation and widespread cell death.
Ongoing and future research
This therapeutic approach is being evaluated for its suitability in humans, Dr. Shapiro said. This includes assessments of its chemical properties, stability, and effects on other organs such as the heart.
Future directions are to test newly developed compounds that have similar action to the compound used in this study, but with highly increased potency and selectivity for KCNQ potassium ion channels in the brain.
###
Acknowledgments
This study was funded by multiple investigator grants from the U.S. Department of Defense through the Congressionally Directed Medical Research Programs.
All experiments were approved by the Institutional Animal Use and Care Committee at UT Health San Antonio and followed the National Institutes for Health’s Guide for Care and Use of Laboratory Animals. The experiments reported here followed the Animal Research: Reporting In Vivo Experiments guidelines.
The researchers are from the Joe R. and Teresa Lozano Long School of Medicine at UT Health San Antonio. Dr. Shapiro is a professor in the Department of Cellular and Integrative Physiology. Dr. Vigil is a postdoctoral fellow and Dr. Robert Brenner an associate professor in the same department. Dr. Jose Cavazos is a professor in the Department of Neurology and assistant dean and director of the South Texas Medical Scientist Training Program. James Lechleiter, Ph.D., a study co-author, is a professor in the Department of Cell Systems and Anatomy, UT Health San Antonio.
Stay connected with UT Health San Antonio on Facebook, Twitter, LinkedIn, Instagram and YouTube.
The University of Texas Health Science Center at San Antonio, now called UT Health San Antonio®, is one of the country’s leading health sciences universities. With missions of teaching, research, healing and community engagement, its schools of medicine, nursing, dentistry, health professions and graduate biomedical sciences have produced 36,500 alumni who are leading change, advancing their fields and renewing hope for patients and their families throughout South Texas and the world. To learn about the many ways “We make lives better®,” visit //www.
https:/
https:/
https:/
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.

