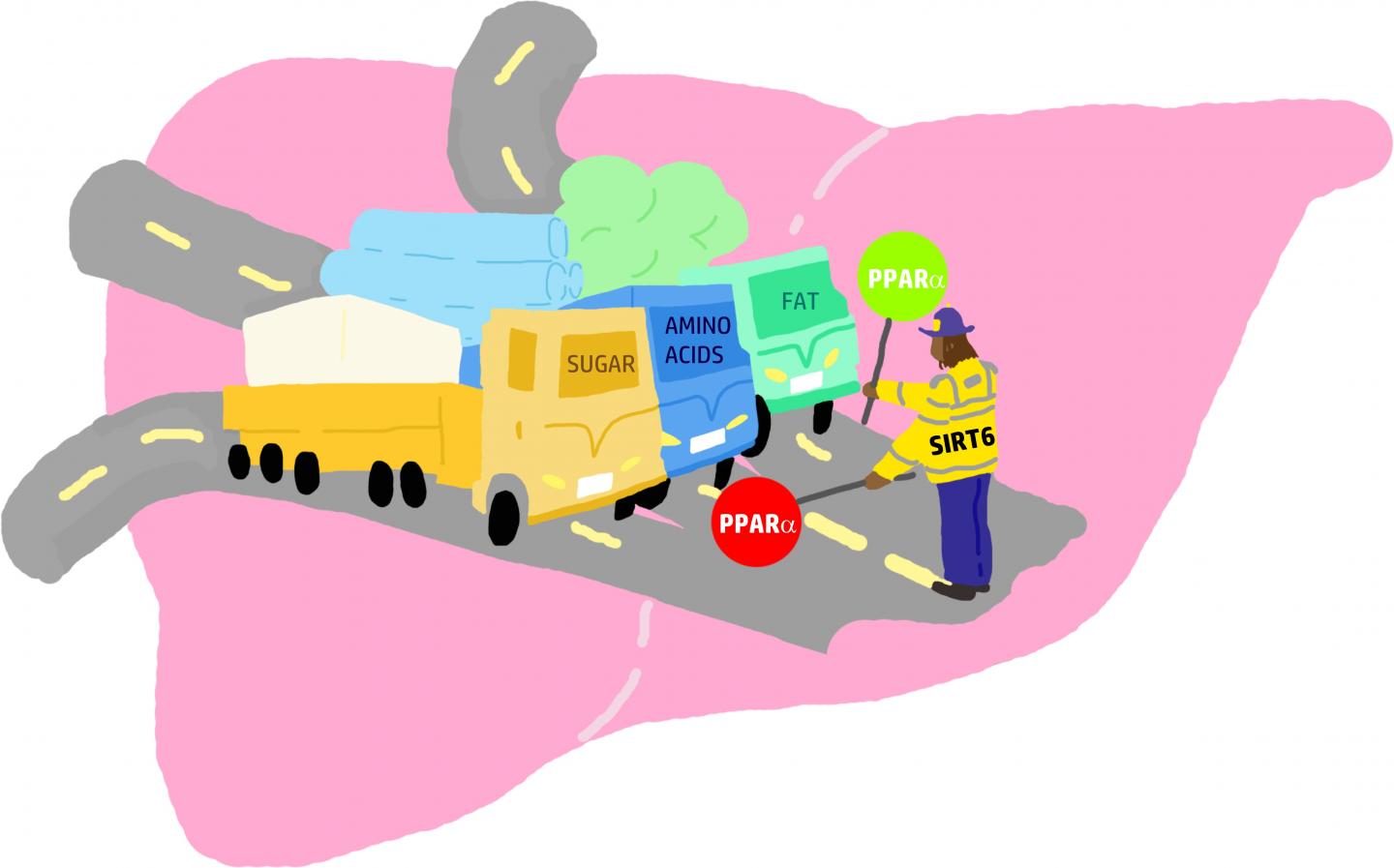
Fatty liver, or hepatic steatosis, which develops when the body produces too much fat or doesn’t metabolize fat efficiently enough, affects around 25% of the global population. Excess fat is stored in liver cells, where it accumulates and can cause fatty liver and other diseases.
In a study just published in the journal Cell Reports, researchers reveal for the first time that SIRT6, a protein involved in regulating many biological processes such as aging, obesity, insulin resistance, inflammation and metabolism, also plays a crucial role in burning and regulating liver fat metabolism.
SIRT6 regulates fat metabolism by activating another protein called peroxisome proliferator-activated receptor alpha (PPAR-alpha). This protein promotes the burning of fat in the liver. “SIRT6 is like a juggler that balances and coordinates between metabolic processes in the body,” says the study’s lead author Prof. Haim Cohen, of Bar-Ilan University’s Mina and Everard Goodman Faculty of Life Sciences. “By working together with PPAR-alpha, SIRT6 can actually send a message to the body to burn more fat. This cooperation is one way in which SIRT6 protects against fatty liver and fatty liver disease, as well as obesity-related damage.”
Previous research by Cohen and colleagues revealed that increased SIRT6 extends lifespan. To test how the protein might also extend healthy lifespan the researchers in this study increased regular SIRT6 levels to counteract the decrease in SIRT6 found in a high fat diet and fatty liver disease. Using a computational tool developed with Prof. Ziv Bar Joseph from Carnegie Mellon University, they looked at different metabolic states, such as fasting and regular diet, and found that a greater expression of SIRT6 leads to the burning of more fat, specifically in the liver.
“Not only does SIRT6 work with PPAR-alpha to prime the body to burn more fat and coordinate fat metabolism in the liver, but it can also regulate other metabolic pathways related to fat in the liver such as cholesterol and triglycerides metabolism,” says Shoshana Naiman, a doctoral student at Bar-Ilan University’s Mina and Everard Goodman Faculty of Life Sciences, who co-authored the study.
###
The team will now attempt to identify therapeutic approaches that can target and activate SIRT6.
This research was supported by grants from the Israel Science Foundation, the U.S.-Israel Binational Science Foundation, and the Sagol Healthy Longevity Center at Bar-Ilan University.
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.

