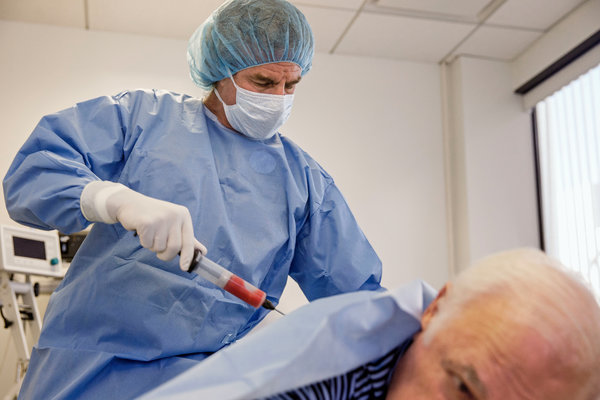
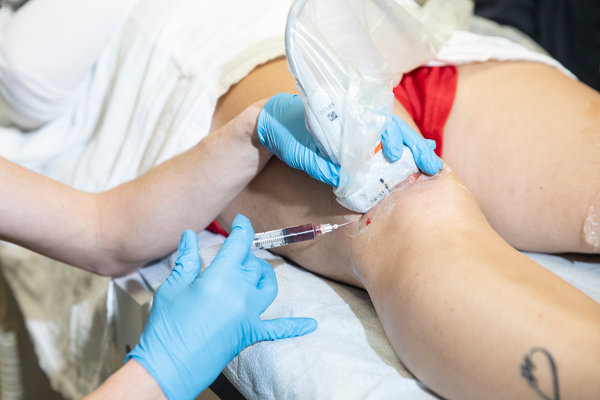
A federal judge on Tuesday issued a permanent injunction against U.S. Stem Cell, a Sunrise, Fla., clinic accused of blinding three patients by injecting a fat extract into their eyes.
The company is just one of hundreds of businesses that have sprung up around the country offering to treat a wide array of illnesses with products they say contain stem cells that have healing and regenerative properties. Medical experts say there is no proof that such treatments work.
Many of those clinics, like U.S. Stem Cell, use extracts from fat. Others use patients’ own bone marrow, and some use cord blood or other birth tissue like amniotic membranes.
Although the injunction applies only to one company, it is widely seen as a warning to others that perform similar procedures.
The injunction stated that U.S. Stem Cell’s fat extracts are legally a drug, subject to regulation by the Food and Drug Administration. The clinic can no longer produce or market the extracts unless it meets the F.D.A.’s standards for good manufacturing practice.
U. S. Stem Cell had claimed that it could treat neurological, autoimmune, orthopedic and degenerative diseases, including Parkinson’s, amyotrophic lateral sclerosis (also known as A.L.S. or Lou Gehrig’s disease), lung and heart disease, back problems, arthritis and other ailments. The clinic had been performing liposuction on patients to suck out belly fat, then processing the fat to extract what it said were stem cells and then injecting the extract back into patients.
The company’s chief scientific officer, Kristin C. Comella, had argued that the extracts contained patients’ own cells, and therefore were not a drug and were exempt from regulation by the F.D.A.
[Like the Science Times page on Facebook. | Sign up for the Science Times newsletter.]
The judge, Ursula Ungaro of United States District Court for the Southern District of Florida, granted the F.D.A. its request for an injunction on June 3. The agency had sought to stop the clinic’s use of the procedure beginning in May 2018.
U.S. Stem Cell said at the time of the initial ruling that it would stop using the fat extracts. But the company also said it would continue offering treatments produced from birth tissues and bone marrow.
In response to the final order issued on Tuesday, the company issued a statement saying it was pleased that the judge had limited the injunction to procedures involving fat extracts.
In that order, Judge Ungaro detailed the steps the company would have to take to resume the procedure using fat cell extracts. U.S. Stem Cell would have to hire, at its own expense, an independent expert to inspect its facility and manufacturing methods, and certify to the F.D.A. that it was complying with the agency’s rules.
The agency would also have to inspect the facility, at the company’s expense. If the company passed the inspection, it would then have to hire an auditor to inspect the facility at least once every six months for two years, and then at least once a year after that.
If the company failed to comply, the F.D.A. could order it to stop manufacturing and recall its products, and could fine it $15,000 for each violation, and $15,000 a day for as long as the violation continues.
The F.D.A. said the injunction “sends a strong message to others” who manufacture unapproved stem cell products.
In a statement, Dr. Norman E. (Ned) Sharpless, the acting F.D.A. commissioner, and Dr. Peter Marks, director of the agency’s biologics center, said: “We know that there are clinics across the country that manufacture or market violative stem cell products to patients, claiming that they don’t fall under the regulatory provisions for drugs and biological products. The F.D.A. has consistently stated that this is not true, and the result of this case proves that.”
The statement also said that the companies have been taking advantage of patients, “many in vulnerable positions with chronic or terminal diseases.”
Legal experts say the judge’s ruling suggests that the F.D.A. is also likely to win an injunction that it has requested against another stem-cell business, Cell Surgical Network, a California company with dozens of affiliates around the country.