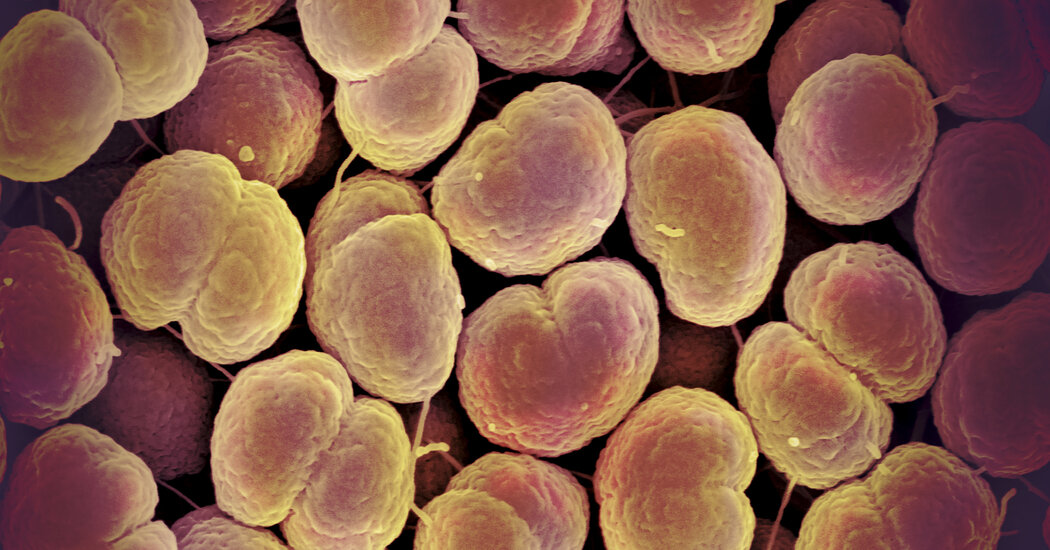
Why It Matters: Gonorrhea is a major global problem.
With more than 82 million new infections recorded worldwide in 2020, gonorrhea is among the most common sexually transmitted diseases. The pathogen, Neisseria gonorrhoeae, spreads through sexual contact to the genitals, rectum and throat.
About half of infected people show no symptoms, but in others gonorrhea can lead to painful joints and burning urination. Left untreated, it can cause infertility and sterility, blindness in infants or even death.
Over the years, the bacterium has found a way to dodge nearly every available antibiotic. It has become resistant to azithromycin and is increasingly resistant to another antibiotic called ceftriaxone, which is now the standard of care.
The most powerful defense combines a shot of ceftriaxone with azithromycin, but some evidence hints that gonorrhea is evolving to sidestep even that treatment.
Zoliflodacin is a new type of antibiotic, boosting hopes that the bacterium will remain susceptible to it for a long time.
“This is a new drug, genuinely solving a problem that really needs to be solved,” said Dr. Manica Balasegaram, executive director of Global Antibiotic Research & Development Partnership, or G.A.R.D.P., a nonprofit that shepherded the drug’s development.
“This doesn’t happen often,” he added.
The Back Story: A clever way to create new antibiotics.
Pharmaceutical companies have largely abandoned antibiotic development as unprofitable. The development of zoliflodacin represents a new model: G.A.R.D.P., which is funded by many Group of 20 countries and the European Union, developed the drug in collaboration with an American pharmaceutical company called Innoviva Specialty Therapeutics.
The nonprofit sponsored the Phase 3 trial of the drug. In exchange, it holds the license to sell the antibiotic in about 160 countries while Innoviva retains marketing rights for high-income countries.
“I’ll go out on a limb and say that’s probably the only way in which we develop antibiotics going forward, because the old model is simply not going to work,” said Ramanan Laxminarayan, a senior research scholar at Princeton University who chairs the G.A.R.D.P. board.
The agreement ensures that the antibiotic will be available and affordable for people in low- and middle-income countries.
“Nobody’s making a boatload of money off treatment of gonorrhea, especially when you’re using a single dose of an oral antibiotic,” said Dr. Jeanne Marrazzo, director of the National Institute of Allergy and Infectious Diseases.
“This is a path forward to solve the dilemma of getting pathways for products that don’t guarantee profits,” Dr. Marrazzo said.
What We Don’t Know: The drug may not cure all cases.
The clinical trial enrolled 925 people in five countries, the largest so far for a gonorrhea treatment. It showed that zoliflodacin was as effective at treating gonorrhea as the combination of ceftriaxone and azithromycin.
The trial was designed to test how well zoliflodacin works in the urogenital tract. Based on previous research, the drug is unlikely to be as effective in the throat and rectum, said Dr. Marrazzo. But “this will give us a pathway to at least address very common infections, particularly in women, worldwide,” she said.
The drugmakers were more sanguine. The numbers of throat and rectal infections were too small to produce firm results, “but we’re very encouraged because they were comparable” to the urogenital tract, said Dr. Margaret Koziel, Innoviva’s chief medical officer.
What’s Next: Scientists will try to prevent resistance.
The more widely a drug is used, the greater the chances that pathogens will find ways to defend against it. In studies, zoliflodacin appears to be effective against a wide range of resistant strains of gonorrhea.
But that does not preclude the possibility that the bacterium may yet evolve to dodge the drug. The partnership’s agreement minimizes that chance: The nonprofit plans to manage how the drug is distributed, and to see that it is used only to treat gonorrhea.
