IMAGE: El Camino Hospital today announced it is the first hospital in California to have successfully performed the new and recently Food and Drug Administration (FDA) approved lung valve treatment for… view more
Credit: Pulmonx Corporation – www.MyLungsMyLife.com
Mountain View, Calif. – March 11, 2019 – El Camino Hospital today announced it is the first hospital in California to have successfully performed the new and recently Food and Drug Administration (FDA) approved lung valve treatment for patients with severe COPD/emphysema, an incurable lung disease that causes extreme breathing difficulty and constant discomfort for patients. The Zephyr® Endobronchial Valve treatment represents a major advancement because it is the first minimally invasive procedure to help emphysema sufferers breathe easier without major surgery.
More than 15 million Americans suffer from COPD, and 3.5 million of those patients have emphysema according to the American Lung Association. Despite using COPD medications, over one million emphysema patients continue to suffer symptoms of hyperinflation, in which air becomes trapped in the lungs and prevents new air from coming in, causing severe shortness of breath. Breathing becomes inefficient and patients have to work very hard just to breathe – making normal activities, like walking, eating or even bathing, difficult. There are few treatment options for most patients with emphysema and there is no cure.
“As one of the first hospitals to offer bronchoscopic lung volume reduction, we are excited to be at the forefront of minimally-invasive lung care,” says Dr. Ganesh Krishna, medical director of the Interventional Pulmonology Program at El Camino Hospital and a doctor at Palo Alto Medical Foundation, part of Sutter Health. “This procedure has the potential to improve breathing, lung function and quality of life for so many people living with emphysema and COPD in California and the Western United States.”
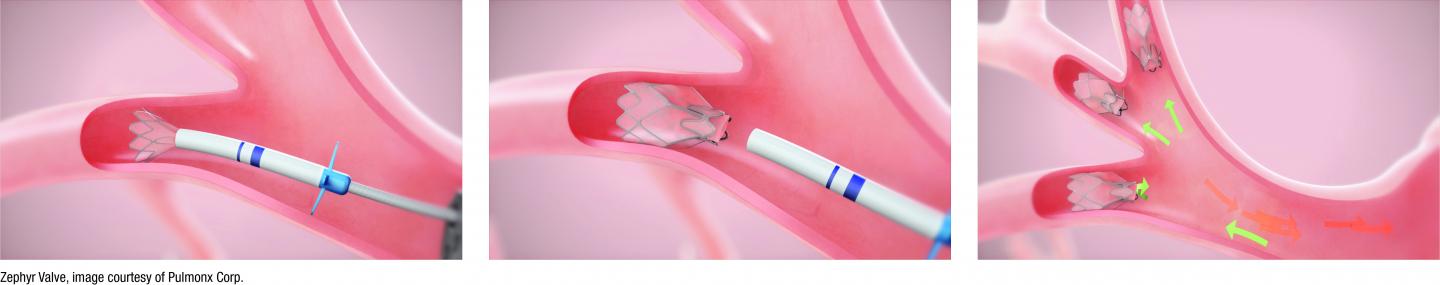
For years, the only treatment options for people with severe COPD were highly invasive treatments such as lung volume reduction surgery or lung transplantation. After clinical trials, including the US approval study LIBERATE which the Taft Center for Clinical Research at El Camino Hospital participated in, the FDA recently approved the Zephyr Endobronchial Valve treatment under their “Breakthrough Devices” status. The one-time procedure is done during a simple bronchoscopy that requires no cutting or incisions. During the procedure, tiny valves are placed in the airways to block off the diseased parts of the lungs where air gets trapped. Keeping air from getting trapped in the diseased parts of the lung allows the healthier parts of the lungs to expand and take in more air. This results in patients being able to breathe easier and have less shortness of breath. Since FDA approval, El Camino Hospital has performed the second highest number of Zephyr Endobronchial Valve procedures in the country.
The Interventional Pulmonology Program at El Camino Hospital is a leader in providing latest, minimally invasive, state-of-the art diagnostic and therapeutic modalities, including offering airway recanalization, bronchial thermoplasty, clinical trials, confocal endomicroscopy, electromagnetic navigation guided (EMN) bronchoscopy, endobronchial ultrasound (EBUS) and transbronchial cryobiopsy. In addition to leading-edge technology and procedures, El Camino Hospital, in collaboration with the Palo Alto Medical Foundation and University of California, San Francisco (UCSF), and Dr. Krishna lead one of only 30 interventional pulmonary fellowship programs in the country.
###
More on the Zephyr Valves:
The Zephyr® Valves were fast-tracked through the FDA’s “Breakthrough Device” status because they “offer bronchoscopic lung volume reduction without surgery and its associated risks.” The FDA’s approval was based on the results of four randomized controlled clinical trials, including the US approval study, LIBERATE. Data from the study showed that implantation of the Zephyr Valves successfully reduced shortness of breath while improving lung function, exercise capacity, and quality of life. 1 These benefits lasted at least one-year post-treatment for patients with severe emphysema. The Zephyr Valves were approved by the FDA in July 2018. Since 2007, more than 15,000 patients have been treated with The Zephyr Valve worldwide. Zephyr Valve treatment is included in emphysema treatment recommendations issued by leading health organizations worldwide, including the Global Initiative for Chronic Obstructive Lung Disease (GOLD) and the UK’s National Institute for Health and Care Excellence (NICE).
About El Camino Hospital
El Camino Hospital is an acute-care, 443-bed, nonprofit and locally governed organization with campuses in Mountain View and Los Gatos, California. Key medical specialties include cancer, heart and vascular, men’s health, mental health and addictions, lung, mother-baby, neuroscience, orthopedic and spine, and urology. The hospital is recognized as a national leader in the use of health information technology and wireless communications. El Camino Hospital has also been awarded the Gold Seal of Approval from The Joint Commission as a Primary Stroke Center as well as three consecutive ANCC Magnet Recognitions for Nursing Care. To learn more visit //www.
1. Criner G, Sue R, Wright S, Dransfield M, Rivas-Perez H, Wiese T et al. A multicenter RCT of Zephyr® Endobronchial Valve Treatment in heterogeneous emphysema (LIBERATE). Am J Resp Crit Care Med. Published online May 22, 2018. https:/
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.

