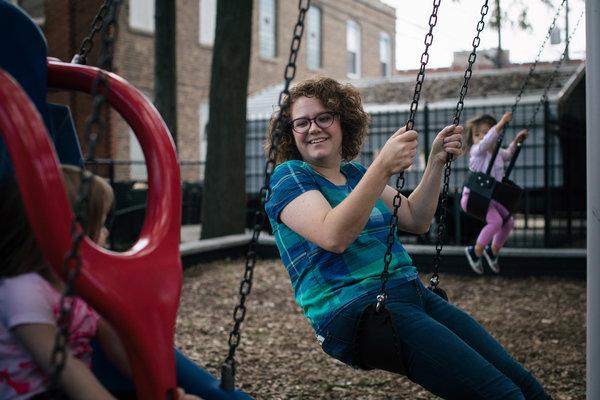Overweight women limited their weight gain with a diet and exercise program during pregnancy, but it did not lower their rate of complications like gestational diabetes.
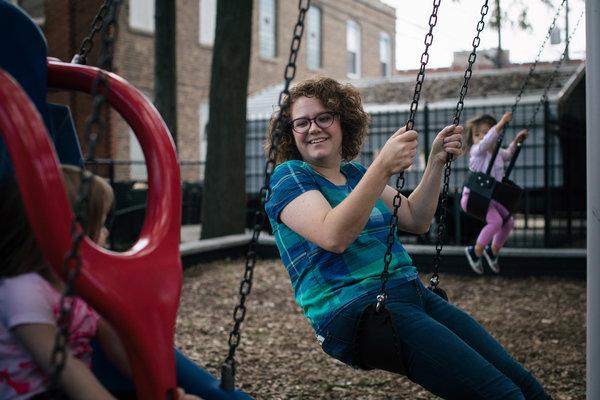
Heather Kinion joined a body weight study at Northwestern in her first trimester and was assigned to work with a nutrition coach.CreditCreditAlyssa Schukar for The New York Times
For years, maternal health experts have worried about a troubling statistic: More than half of all pregnant women in America are overweight or obese when they conceive, putting them and their children at a higher risk of developing diabetes and other health problems.
So about a decade ago, the federal government launched a multimillion-dollar trial to see whether diet and exercise could help overweight women maintain a healthy weight during their pregnancies and potentially reduce their rate of complications. On Thursday, the findings were announced, and the results were mixed: Starting a diet and exercise program around the beginning of their second trimesters helped many women avoid excess weight gain during their pregnancies. But it did not lower their rate of gestational diabetes, hypertension and other adverse outcomes.
Experts said the research was both encouraging and sobering. It confirmed that overweight and obese women can safely limit their pregnancy weight gain with lifestyle interventions. But it also suggests that to improve obstetric outcomes and the health of their babies, women who are carrying extra weight may need to make significant lifestyle changes before they conceive, said Dr. Alan Peaceman, the chief of maternal fetal medicine at Northwestern University Feinberg School of Medicine and the lead investigator of the study, which was published in Obesity.
“This is a problem that is more important now than it’s ever been, and it needs to be addressed,” he added. “We are going to have to start talking to women who are overweight or obese even before pregnancy and explain to them the risk of that weight on a potential pregnancy.”
[Listen to Dr. Peaceman’s podcast on high B.M.I. and pregnancy weight gain.]
The new research comes at a critical time. Decades ago, health authorities routinely urged pregnant women to put on enough weight to lower their odds of having underweight babies. But when the obesity epidemic took off in the 1980s and ’90s, it spared almost no population, including pregnant women. Research by the Centers for Disease Control and Prevention found that the prevalence of obesity among pregnant women climbed by 69 percent from 1993 to 2003. Today about 26 percent of women are overweight when they enter pregnancy and 25.6 percent are obese, according to the latest C.D.C. data.
Women in those groups are more likely to exceed the recommended amount of weight gain during pregnancy and to retain that weight postpartum. Among the complications they are more likely to experience are longer labors, abnormally large babies, hypertension and cesarean deliveries. Obese women also have higher rates of gestational diabetes, miscarriages and preterm births. And a number of studies show that their children have increased rates of obesity and Type 2 diabetes.
In 2009, the Institute of Medicine issued a report outlining the amount of weight that women should gain during their pregnancies based on their body mass index. Women in the normal weight category should gain between 25 and 35 pounds, the guidelines state, while those who are overweight should add 15 to 25 pounds. Obese women are encouraged to gain no more than 20 pounds during pregnancy.
Over the years, a number of studies looked at whether lifestyle changes could improve health outcomes for expectant mothers with high B.M.I.s. But many of the studies were small, not very rigorous or of poor quality, so the National Institutes of Health set out to fund a large and definitive study in a diverse group of women. The resulting study recruited 1,150 overweight and obese women at seven clinics across the country and randomly assigned them to a control group or an intervention group that followed a variety of diet and exercise strategies. The women were all between nine and 15 weeks pregnant when they joined the study.

Ms. Kinion and her daughter, Julia, 2, play in their Chicago neighborhood, Avondale.CreditAlyssa Schukar for The New York Times
The subjects included women like Heather Kinion, 39, who lives in Chicago and works for a quilting magazine. Ms. Kinion was slightly overweight when she got pregnant in the fall of 2015. She joined the study at Northwestern in her first trimester and was assigned to work with a nutrition coach who instructed her to track her food intake with a smartphone app. Ms. Kinion ate no more than 2,300 calories per day, replaced soft drinks with tea, and cut back on sugary treats like ice cream, cinnamon rolls and milk shakes. She added more fruits and vegetables to her diet and tried to exercise and walk more.
Ms. Kinion gained about 20 pounds during her pregnancy — which was in the recommended range of weight gain — and delivered a healthy baby, named Julia, in 2016.
“It was super effective,” she said of the program. “Within two weeks of my daughter being born I was down to a weight that I think was 10 pounds lower than pre-pregnancy.”
Ultimately, the researchers found that the women in the diet and exercise arm of the study gained on average four pounds fewer than those in the control group. They were 48 percent less likely to exceed the Institute of Medicine’s recommended amount of pregnancy weight gain.
Yet for most women, the intervention did not work. About 68.6 percent of women in the diet and exercise group exceeded the recommended amount of weight gain, compared to 85 percent of women in the control arm. At the end of the study, their rate of major pregnancy complications did not differ.
“One of our prevailing suspicions is that when we started with the intervention at the beginning of the second trimester it was already too late,” Dr. Peaceman said. “It’s possible the adverse outcomes were already influenced by weight gain before that time.”
Dr. Emily Oken, a maternal health expert at Harvard Medical School who was not involved in the research, said that future studies could look at the impact of assigning overweight women to make lifestyle changes before they get pregnant. She also speculated that the average reductions in weight gain that occurred in the new study might not be large enough to have any real impact for many women.
“It’s not clear that these small differences in weight gain result in differences in outcomes for the baby,” Dr. Oken said.
Another expert on maternal health, Dr. Patrick Catalano, said it was clear from other studies that public health efforts should focus on reaching women to help them improve their health long before they get pregnant.
“My belief is that this has to be a life course approach — it can’t just be something we try when women are already 14 weeks into pregnancy,” said Dr. Catalano, a senior research investigator at the Mother Infant Research Institute at Tufts Medical Center. “If you come into a pregnancy normal weight then statistically you’re at a decreased risk for having a lot of complications. If the goal is to try to improve pregnancy outcomes for both the mother and her offspring, then we need to start early.”
Anahad O’Connor is a staff reporter covering health, science, nutrition and other topics for Science Times and the Well blog. He is also a bestselling author of consumer health books such as “Never Shower in a Thunderstorm” and “The 10 Things You Need to Eat.”
Advertisement