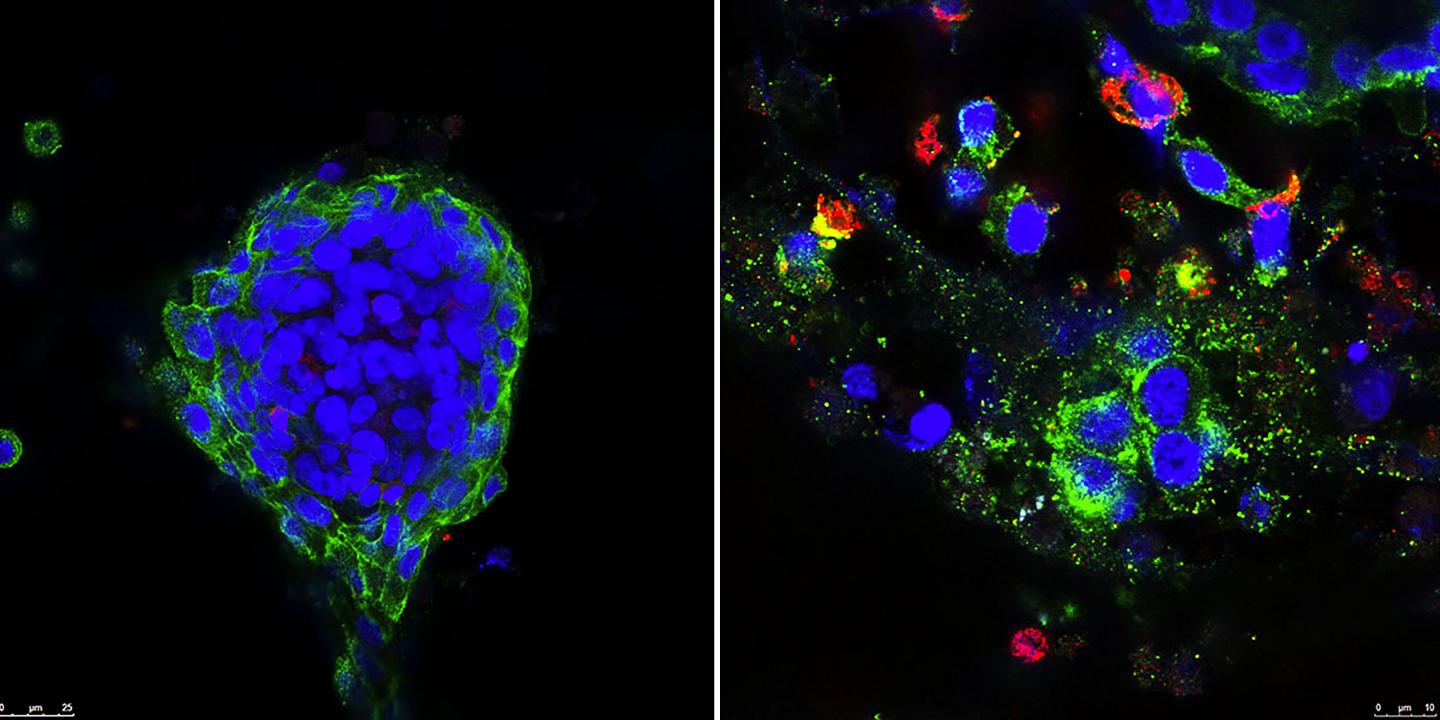
IMAGE: Left: Untreated patient breast cancer tissue was grown in three-dimensional culture. Right: Cancer tissue was treated with BCL-2 inhibitor and antidiabetic drug. Green color indicates the cancer cells, and red… view more
MYC, a gene with high cancer-initiating potential, is overexpressed in over 40% of breast cancers. While MYC programs breast cancer cells to build more macromolecules (anabolic metabolism) it also creates a metabolic vulnerability by making them more sensitive to a type of cell death known as apoptosis. Research Director Juha Klefstrom, PhD, University of Helsinki, Finland, has worked for a long time to exploit this apoptosis-sensitizing effect of MYC in the battle against the cancer.
Klefstrom and his research group found that, because of this vulnerability, cancer cells can be attacked with a “drug cocktail” that includes the diabetes drug metformin and venetoclax, a BCL-2 protein inhibitor that can induce apoptosis in cancer cells. The group identified metformin in a search for drugs that could boost the apoptosis-inducing action of venetoclax. Venetoclax has been approved to treat certain leukemias but not yet for the treatment of breast cancer.
“This drug combo exploits specific metabolic vulnerabilities that high levels of MYC creates in tumor cells. Metformin and venetoclax, when given together, killed breast tumor cells in culture and blocked tumor growth in breast cancer animal models. Furthermore, the drugs efficiently killed authentic breast cancer tissue donated by breast cancer patients. The breast cancer samples were obtained fresh from surgeries performed in Helsinki University Hospital”, Dr. Klefstrom says.
However, the researchers soon discovered that the metformin plus venetoclax treatment only held tumors in check as long as the mice with implanted breast tumors were actively being treated with the drugs; once the treatment was stopped, the tumors grew back. The study shows that tumors were initially filled with tumor-killing lymphocytes; however, after the treatment they largely vanished and the remaining killer cells expressed PD-1, a marker of immune cell exhaustion.
To help the immune cells better fight the tumor, the researchers developed a new treatment strategy. First, they hit breast tumors with apoptosis-inducing drugs metformin and venetoclax to reduce the tumor size and to wake up killer lymphocytes. After the primary tumors were surgically removed, the mice were then treated with a triple combination: metformin, venetoclax and a PD-1-targeted antibody, which is used in immunotherapies to keep killer cells active long-term.
“With this combination the survival of mice carrying implanted tumors was extended dramatically in comparison to mice that were treated with only single or double combinations”, Klefstrom states.
Klefstrom highlights that this is a wonderful example of a translational study fundamentally aimed at taking research from bench to bedside. The key people from the University of Helsinki and Helsinki University Hospital (HUS) – basic researchers, pathologists, surgeons and oncologists – were all involved at the earliest stages of the study.
The first author of the study Dr Heidi Haikala notes: “It’s quite amazing how we’ve been able to bring a discovery from the lab bench all the way to the doors of the cancer clinics within the time frame of one PhD project. We are very excited about our findings and hope that they will translate to benefit breast cancer patients.”
“This is a great example of how scientists in academia, leveraging highly specialized tumor models and applying their unique insights, can contribute to the discovery of potential new treatments for people with cancer. It is also a testament to the great research being done in smaller countries like Finland,” states Joel Leverson, Ph.D., a Senior Scientific Director at AbbVie and one of the senior authors in the study.
“We finally have a drug combination that efficiently exploits MYC’s apoptotic function and most importantly, these drugs can be tested in the clinic in real patients. We are currently working hard towards this next step.”, Klefstrom concludes.
###
The collaborative study involved Juha Klefstrom, Satu Mustjoki and Timo Otonkoski groups at the University of Helsinki, FIMM, HUSLAB pathology, Helsinki University Hospital/HUS breast cancer surgery unit and HUS Comprehensive Cancer Center. Other key collaborators included University of Wurzburg, Germany, Oncotest GmbH (now part of Charles River Laboratories Inc.) and AbbVie, North Chicago.
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.

